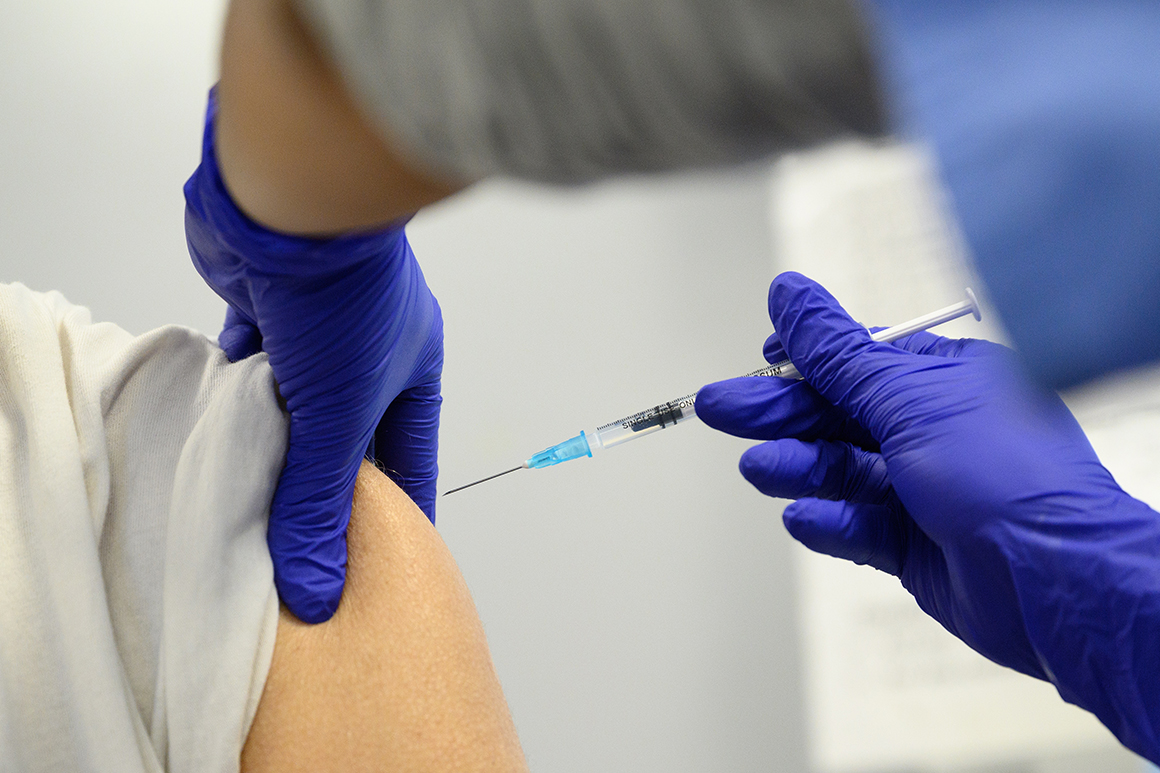
The CDC’s impartial vaccine advisers had voted 11-0 to permit all adults to get Covid-19 boosters of Pfizer and Moderna photographs.
The panel stated that every one American adults “might” go for a booster relying on their particular person advantages and dangers, whereas these 50 and older “ought to” get boosted. The wording distinction acknowledges the practicality of an age-based advice whereas additionally recognizing the skinny information on the advantages of boosting younger and in any other case wholesome adults.
The earlier federal booster suggestions lined anybody 65 and older, adults with sure underlying well being situations and adults whose jobs enhance their possibilities of contracting or spreading Covid, so long as all had been at the least six months faraway from their second doses. Individuals who’d acquired the Johnson & Johnson vaccine had been eligible for any of the boosters at the least two months after inoculation.
The brand new suggestions are supposed to deal with what many states have stated when leapfrogging the federal authorities on booster entry: Earlier standards had been so complicated that even eligible people weren’t scheduling their photographs.
“The pursuit of precision creates confusion,” stated Nirav Shah, the top of Maine’s Heart for Illness Management and Prevention.
Even the CDC’s checklist of underlying situations that might make Covid extra harmful has modified in the previous couple of weeks, with the company including former people who smoke and people with temper problems to at-risk teams.
“I’m undecided I can sustain with who’s eligible and who’s not eligible,” stated Grace Lee, the advisory committee chair.
One of many largest points the CDC panel has weighed within the final 5 months has been the affiliation between an inflammatory coronary heart situation referred to as myocarditis and the 2 messenger RNA vaccines from Pfizer and Moderna. The facet impact, whereas uncommon, has predominantly affected adolescent and younger grownup males below 30 and was the driving consider its and FDA’s personal advisory panel’s efforts to restrict boosters to solely older and sicker People.
FDA and CDC advisers had been reluctant to green-light boosters for all adults in September when first introduced with the request from Pfizer. They pushed again on permitting the doses for 16- and 17-year-olds, who had been already eligible for the grownup dosage, due to the dearth of security information, they usually’ve usually been averse to recommending additional doses for younger and in any other case wholesome folks.
However the seven-day transferring common of Covid-19 circumstances has jumped 21 p.c over the past two weeks, and a few states are overtly fretting that their hospital programs might quickly be overrun with circumstances across the holidays. Some Biden well being officers consider the upward pattern might have been squashed if extra folks had gotten their booster photographs sooner.
Pfizer did not report any circumstances of myocarditis noticed in its booster trial, however the CDC vaccine security group’s Tom Shimabukuro stated the company has acquired 54 preliminary experiences of myocarditis or pericarditis in individuals who acquired boosters, 12 of which have been confirmed.
That inhabitants displays the inhabitants eligible for boosters, Shimabukuro stated, so that they’re older adults relatively than the adolescents and younger those who have dominated circumstances of the irritation after the second messenger RNA vaccine dose. Due to that, he stated, it is too quickly to attract conclusions in regards to the prevalence of myocarditis after booster administration.
Whereas proof is rising that Moderna’s main vaccine sequence poses a higher danger for myocarditis than Pfizer’s, the Moderna booster dose is half the scale of the primary two doses administered within the sequence, elevating the query of whether or not that smaller quantity is correlated to a decrease danger for the hostile occasion.
Lee acknowledged that some suppose the advisory committee hasn’t moved shortly sufficient to make vaccine suggestions however famous that Friday’s assembly was the twenty second public deliberation on Covid inoculations, a course of she stated is much more necessary throughout a pandemic to domesticate public belief.
Earlier within the day, Colorado Democratic Gov. Jared Polis, who’s usually criticized FDA and CDC for seemingly slow-walking booster and pediatric vaccine choices, panned the method as soon as once more.
“Whereas it has been irritating to look at an unelected board delay needed boosters for People, at the moment I’m glad to see the FDA lastly take the suitable step ahead in additional defending all People from COVID-19,” he stated in an announcement.
The CDC panel’s Friday choice raises one more query: When will they revise the advice to strongly urge all adults all the way down to age 18 to get boosted?
“I really feel like we have had a transferring purpose publish right here,” stated committee member Lynn Bahta of the Minnesota Division of Well being.