WASHINGTON (AP) — U.S. regulators on Friday moved to open up COVID-19 booster photographs to all adults, increasing the federal government’s effort to get forward of rising coronavirus instances that consultants concern might snowball right into a winter surge as hundreds of thousands of People journey for the vacations.
Watch the vote within the participant above.
The Meals and Drug Administration’s determination stands to simplify what has been a complicated listing of who’s eligible by permitting anybody 18 or older to decide on both a Pfizer or Moderna booster six months after their final dose — no matter which vaccine that they had first. The transfer got here after a couple of dozen states had began providing boosters to all adults.
“We heard loud and clear that individuals wanted one thing less complicated — and this, I feel, is straightforward,” FDA vaccine chief Dr. Peter Marks advised The Related Press.
There’s yet one more step earlier than the strategy turns into official: The Facilities for Illness Management and Prevention should conform to broaden Pfizer and Moderna boosters to even wholesome younger adults. Its scientific advisers have been set to debate it later Friday.
If the CDC agrees, tens of hundreds of thousands extra People might have three doses of safety earlier than the brand new yr. Anybody who obtained the one-dose Johnson & Johnson vaccine can already get a booster.
All three COVID-19 vaccines used within the U.S. provide sturdy safety towards extreme sickness together with hospitalization and dying with out boosters, however safety towards an infection can wane with time. Beforehand, the federal government had cleared boosters of Pfizer’s vaccine, in addition to the same Moderna vaccine, just for susceptible teams together with older People and folks with power well being issues.
However Pfizer final week requested the FDA to broaden that call to everybody, citing new knowledge from a examine of 10,000 folks. In the end, the FDA determined there was sufficient proof, from research and real-world use of boosters, to again the enlargement for each Pfizer and Moderna.
The transfer comes as new COVID-19 instances have climbed steadily during the last two weeks, particularly in states the place colder climate is driving folks indoors. Some states didn’t watch for federal officers to behave and opened boosters to all adults.
Marks stated he understood why some governors obtained out forward of the FDA.
“We’re going into a chilly season, instances going up, excessive journey season, folks indoors sharing good vacation instances collectively,” he stated. “They most likely noticed the specter of what might occur right here, and have been attempting — nicely intentioned — to do one thing.”
Boosters for everybody was the Biden administration’s unique purpose. However in September, a panel of FDA advisers voted overwhelmingly towards that concept primarily based on the vaccines’ continued effectiveness in most age teams. As an alternative, they endorsed an additional Pfizer dose just for essentially the most susceptible.
Final month, backed by its advisory panel, the FDA cleared Moderna boosters — utilizing a dose half as large as the primary two photographs — for a similar susceptible teams.
However there was some frustration contained in the White Home and amongst allies of the president that the lengthy and public regulatory course of contributed to misinformation and confusion across the boosters.
Administration officers, together with Dr. Anthony Fauci, continued making the case for utilizing boosters extra broadly, noting that even milder infections in youthful folks could cause “lengthy COVID” and different issues.
“I don’t know of another vaccine the place we solely fear about preserving folks out of the hospital,” Fauci stated Wednesday.
However the administration had pledged that the choice would fall to scientists. This time round, the FDA didn’t seek the advice of its advisers, saying scientific points surrounding Pfizer’s and Moderna’s boosters “don’t increase questions that will profit from extra dialogue.”
Regulators concluded the general advantages of added safety outweighed dangers of uncommon negative effects from Moderna’s or Pfizer’s vaccine, equivalent to a kind of coronary heart irritation seen largely in younger males.
Pfizer and its German companion BioNTech argued that broader boosters might assist tamp down infections at a important interval.
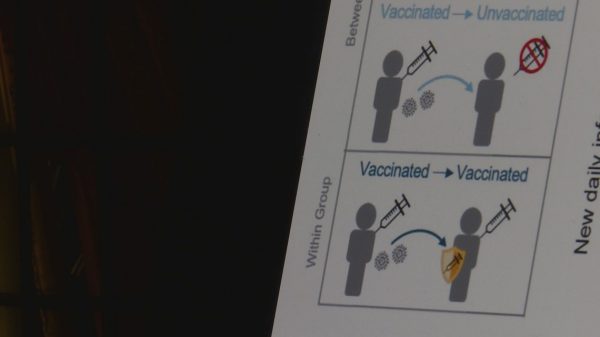
The businesses studied 10,000 adults of all ages and located {that a} booster restored safety towards symptomatic infections to about 95 % even whereas the extra-contagious delta variant was surging. It’s too quickly to know if that top stage of safety will last more after a 3rd shot than after the second. BioNTech CEO Ugur Sahin stated the businesses will rigorously monitor that.
Britain just lately launched real-world knowledge exhibiting the identical leap in safety as soon as it started providing boosters to middle-aged and older adults, and Israel has credited widespread boosters for serving to to beat again one other wave of the virus.
Greater than 195 million People are totally vaccinated, outlined as having obtained two doses of the Pfizer or Moderna vaccines or the single-dose Johnson & Johnson vaccine. Greater than 30 million have already got obtained a booster.
Earlier than the enlargement, individuals who obtained the Pfizer or Moderna vaccinations have been eligible for a 3rd dose in the event that they’re aged or at excessive danger of COVID-19 due to well being issues, their jobs or their residing situations. As a result of a single J&J shot hasn’t confirmed as efficient as its two-dose opponents, any J&J recipient can get a booster at the least two months later.
Individuals who don’t meet the standards have been in a position to get an additional shot as a result of many vaccine websites don’t verify {qualifications}.
The FDA beforehand dominated that individuals getting a booster can obtain a unique model from the vaccine they obtained initially.
Some consultants fear that every one the eye to boosters could hurt efforts to achieve the 60 million People who’re eligible for vaccinations however haven’t gotten the photographs. There’s additionally rising concern that wealthy international locations are providing widespread boosters when poor international locations haven’t been in a position to vaccinate greater than a small fraction of their populations.
“When it comes to the No. 1 precedence for decreasing transmission on this nation and all through the world, this stays getting folks their first vaccine collection,” stated Dr. David Dowdy of Johns Hopkins Bloomberg College of Public Well being.
Related Press author Zeke Miller contributed.