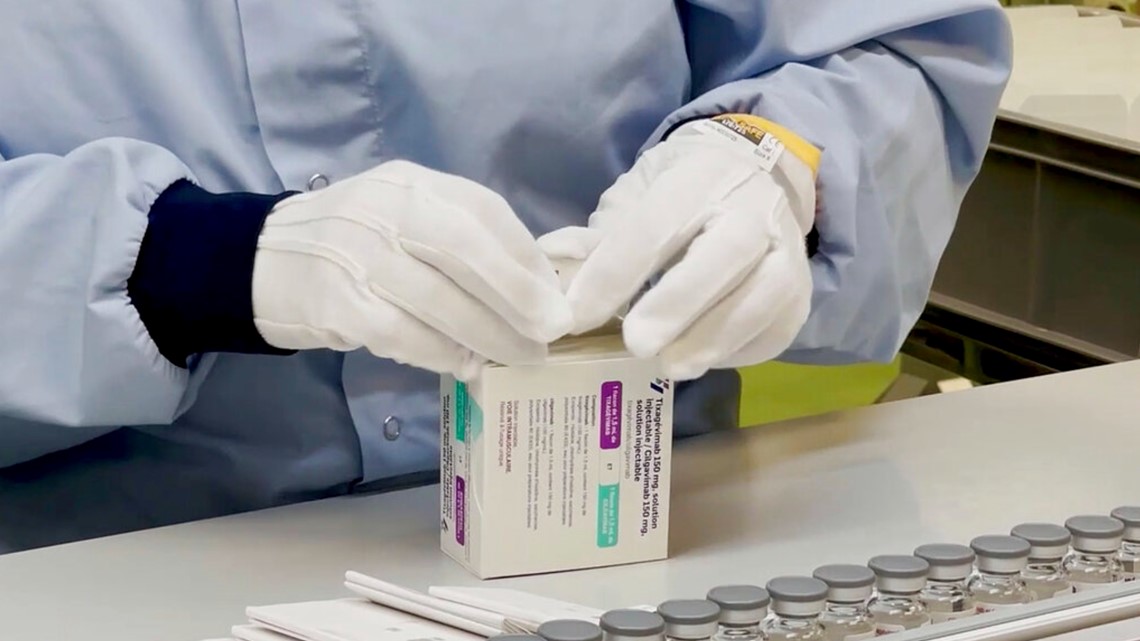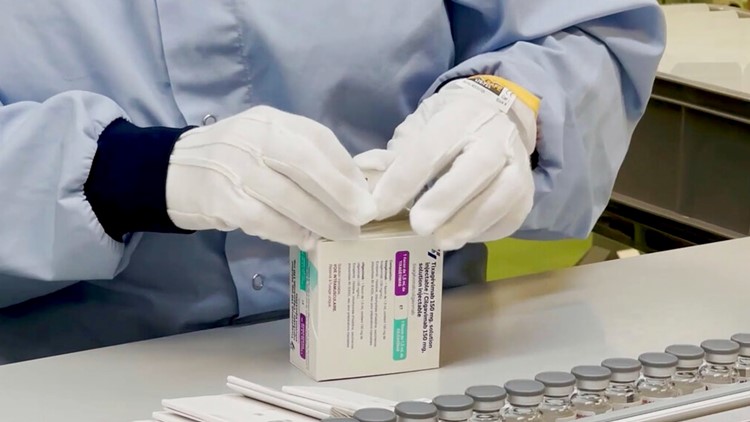
AstraZeneca’s Evusheld is allowed by the FDA solely as a long-term safety measure in individuals who have elevated vulnerability to the coronavirus.
WASHINGTON — Federal well being officers on Wednesday licensed a brand new COVID-19 antibody drug for individuals with critical well being issues or allergic reactions who can’t get enough safety from vaccination.
Antibody medication have been a normal remedy for treating COVID-19 infections for over a yr. However the AstraZeneca antibody drug cleared by the Meals and Drug Administration is completely different. It is the primary supposed for long-term prevention in opposition to COVID-19 an infection, moderately than a short-term remedy.
Individuals who may benefit from the antibody drug embrace most cancers sufferers, organ transplant recipients and folks taking immune-suppressing medication for circumstances like rheumatoid arthritis. Well being consultants estimate about 2% to three% of the U.S. inhabitants falls into that group.
“These individuals nonetheless should shelter in place as a result of they’re at actually excessive threat of extreme illness and dying,” mentioned Dr. David Boulware of the College of Minnesota, forward of the announcement. “So having this remedy will allow loads of them to get again to their regular lives.”
Particularly, the FDA licensed the AstraZeneca drug known as Evusheld for adults and kids 12 and older whose immune programs have not responded adequately to COVID-19 vaccines or have a historical past of extreme allergic reactions to the photographs. Regulators mentioned the required two antibody injections could also be efficient at stopping COVID-19 infections for six months.
Like comparable medication, AstraZeneca’s delivers laboratory-made variations of human antibody proteins, which assist the immune system combat off viruses and different infections.
The FDA and different well being authorities have burdened that antibody medication will not be an alternative choice to vaccines, that are the best, long-lasting and economical type of virus safety. Antibody medication are tough to fabricate and infrequently value greater than $1,000 per dose in contrast with vaccines which might be usually underneath $30 per shot.
The FDA has licensed three different antibody therapies from Regeneron, Eli Lilly and GlaxoSmithKline, with the U.S. authorities buying tons of of 1000’s of doses. All require an IV or injection. They’re used to deal with individuals with latest infections who’ve the best threat of progressing to extreme COVID-19 due to different well being points. Two can be utilized to forestall an infection after a potential coronavirus publicity.
AstraZeneca’s drug can be used in a different way— solely as a long-term safety measure in individuals who have elevated vulnerability to the virus.
In an organization research, individuals who acquired Evusheld had a 77% decrease threat of an infection than individuals who acquired a dummy shot over six months, the FDA mentioned.
The Related Press Well being and Science Division receives assist from the Howard Hughes Medical Institute’s Division of Science Training. The AP is solely chargeable for all content material.