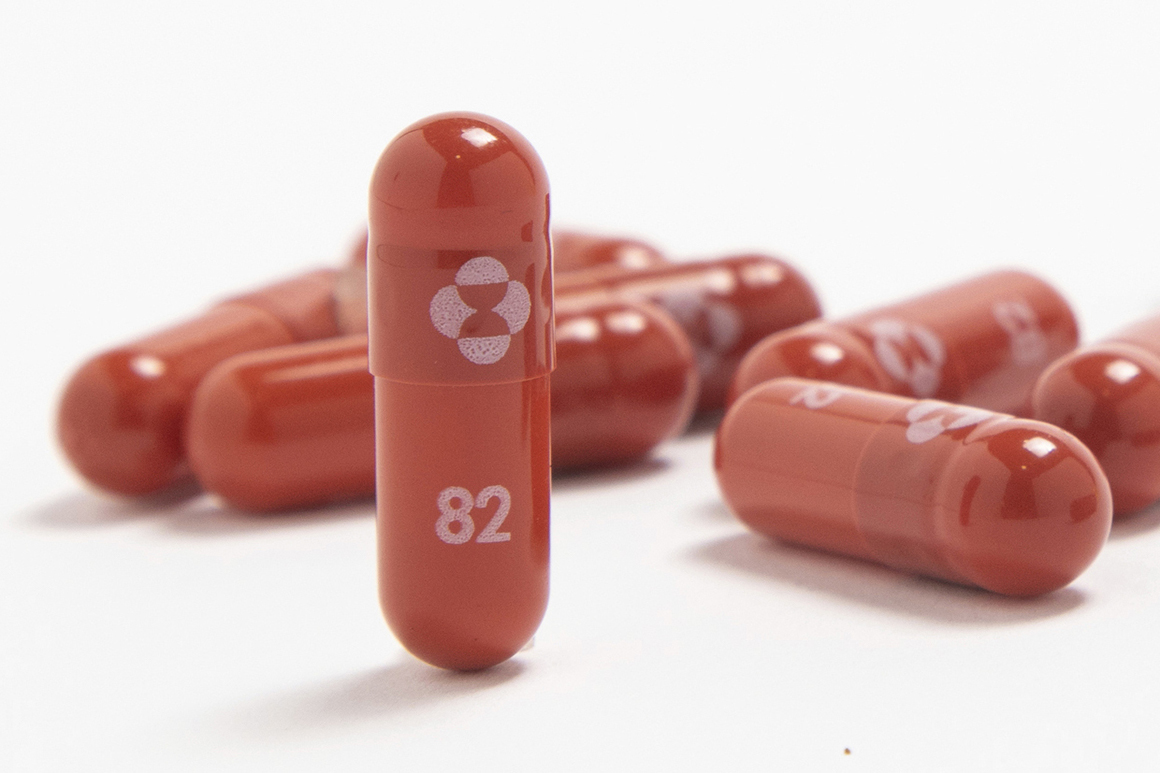
Sufferers will want a prescription and will begin taking the tablet inside 5 days of experiencing signs. The remedy is taken twice a day for 5 days.
Scientific trials confirmed that Merck’s tablet decreased the chance of hospitalization and demise from the virus by 30 p.c.
“As new variants of the virus proceed to emerge, it’s essential to develop the nation’s arsenal of COVID-19 therapies utilizing emergency use authorization, whereas persevering with to generate further knowledge on their security and effectiveness,” stated Patrizia Cavazzoni, the director of the FDA’s Heart for Drug Analysis and Analysis.
The FDA on Wednesday licensed the nation’s first antiviral tablet for Covid — Pfizer’s Paxlovid — which the company stated ought to be an necessary device for serving to scale back the present pressure on hospitals flooded with Covid sufferers and grappling with well being employee shortages. In scientific trials, Pfizer’s remedy decreased the chance of hospitalization and demise by 89 p.c when taken three days after the onset of signs.
On a press name Thursday, high FDA officers stated that the Merck drug, whose effectiveness is considerably decrease than Pfizer’s, is advisable for sufferers for whom different therapies aren’t obtainable.
However they insisted that docs ought to determine which tablet to prescribe on a case by case foundation, noting that Pfizer’s drug may work together badly with another drugs and is not protected for folks with extreme liver or kidney illness.
For each medicine, harassed John Farley, the director of the Workplace of Infectious Ailments within the FDA’s Heart for Drug Analysis and Analysis, “their identified and potential advantages outweigh their identified and potential dangers.”
The authorizations come as Omicron has been detected in all 50 states, resulting in a surge in new infections. Greater than 60,000 Individuals are presently hospitalized with Covid-19, in accordance with the Facilities for Illness Management and Prevention, and the nation is recording greater than 160,000 new infections daily.
The authorizations come as Omicron has been detected in all 50 states, resulting in a surge in new infections. Greater than 60,000 Individuals are presently hospitalized with Covid-19, in accordance with the Facilities for Illness Management and Prevention, and the nation is recording greater than 160,000 new infections daily.
These tablets, which will be taken at dwelling, are anticipated to succeed in much more folks than monoclonal antibody medicine, that are sometimes infused in a scientific setting, and had, till this week, been the one licensed remedy for Covid-19 sufferers who aren’t hospitalized.
The Biden administration has already bought 3.1 million programs of molnupiravir and 10 million programs of Paxlovid.
Katherine Ellen Foley contributed to this report.