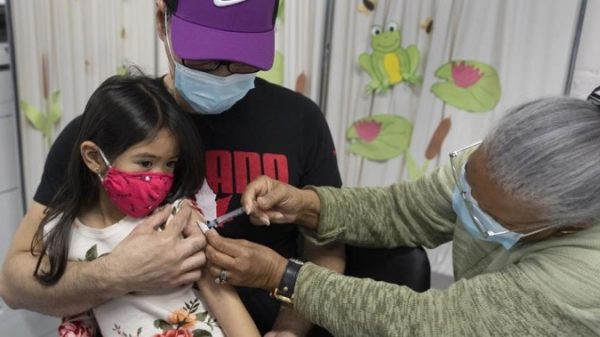
The Pfizer/BioNTech COVID-19 vaccine showed 90.7% efficacy against the coronavirus in a clinical trial of children 5 to 11 years old, the U.S. drugmaker said on Friday.
Sixteen children in the trial who had received a placebo got COVID-19, compared with 3 who were vaccinated, Pfizer said in briefing documents submitted to the U.S. Food and Drug Administration.
Because more than twice as many children in the 2,268-participant trial were given the vaccine than placebo, that equates to better than 90% efficacy.
Read more:
Pfizer asks Health Canada to approve COVID-19 vaccines for children aged 5-11
Pfizer’s clinical trial in those 5 to 11 years old was not primarily designed to measure efficacy against the virus. Instead, it compared the amount of neutralizing antibodies induced by the vaccine in the children to the response of older recipients in their adult trial.
Based on those results, Pfizer and BioNTech said last month that their COVID-19 vaccine induced a robust immune response in the children.
Outside advisors to the FDA are scheduled to meet on Tuesday to vote on whether to recommend that the agency authorize the vaccine for that age group.
Read more:
Pfizer COVID-19 vaccine – How the shot differs for kids and adults
The Pfizer/BioNTech vaccine already has U.S. regulatory authorization for people who are at least 12 years old, including full FDA approval in August for those 16 and up.
Around 190 million people in the United States are fully vaccinated, including more than 11 million who are 12 to 17 years old that have received the Pfizer vaccine.
If the FDA authorizes the vaccine for children 5 to 11 years old, a group of advisors to the U.S. Centers for Disease Control and Prevention will meet on Nov. 2 and 3 to make recommendations to the agency on how the shots should be administered. Most states wait for the CDC to sign off on recommendations for vaccines before they begin administering shots.
View link »