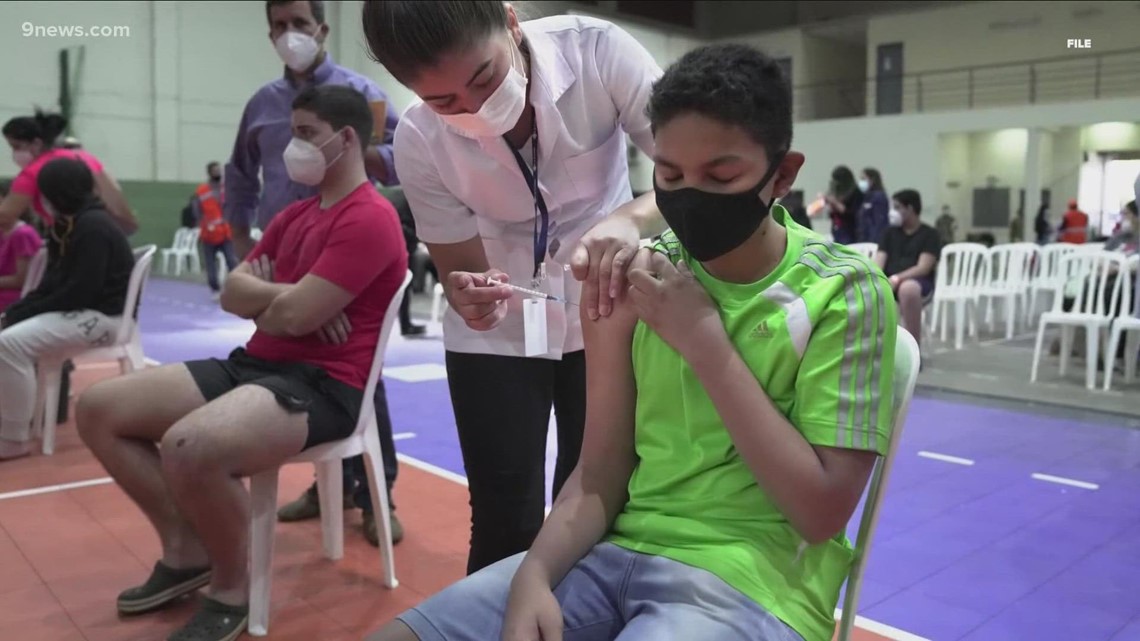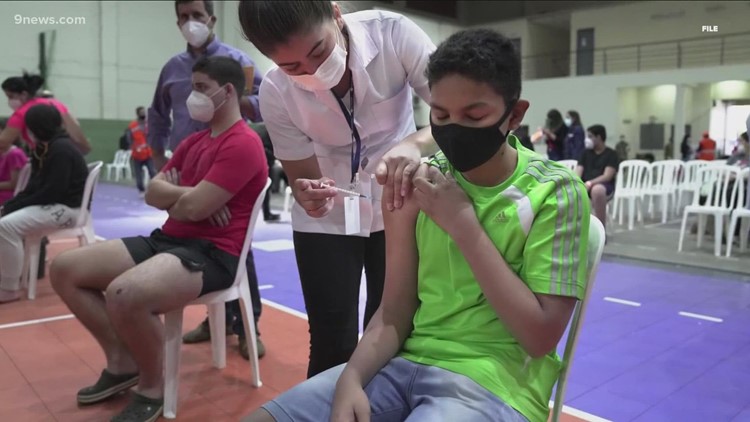
The FDA panel recommending the Pfizer COVID shots for children said the benefits of the vaccine for kids outweigh the risks to kids 5 to 11.
COLORADO, USA — A Food and Drug Administration (FDA) advisory panel has recommended Pfizer’s COVID vaccine for kids younger than 12 – an announcement that could impact more than 28 million children across the country.
The panel said the benefits of the vaccine for kids outweigh the risks in children 5 to 11.
Dr. Richard Besser, the former acting director of the Centers for Disease Control and Prevention, said there have been nearly 2 million cases, more than 8,000 hospitalizations and more than 150 deaths in this age group.
Besser expects this vaccine for children to be recommended to part of this age group – if not the entire age group.
Parents have a decision to make when the vaccine is approved for children under 12, if and when the decision comes down from the FDA.
> Watch: 9Health Expert Dr. Payal Kohli answers questions on COVID vaccines for kids
In Colorado, public health leaders are working with local public health partners, pediatricians and healthcare systems to prepare for this rollout – and they are also making bigger plans.
“We are also working with community partners to ensure that we have large scale events in areas where the community can easily access this vaccine,” said COVID-19 Incident Commander Scott Bookman with the Colorado Department of Public Health and Environment. “We’ve got a robust planning process underway and we’ll provide more information on that next week.”
After the FDA’s recommendation, Gov. Jared Polis released a statement about what the recommendation means for the children and families of Colorado.
“Our youngest Coloradans will soon have a tool to protect themselves from this dangerous virus so they can enjoy in-person learning without fear and so they can safely visit their grandparents and friends,” Polis said. “Child COVID-related hospitalizations are preventable once kids have the ability to roll up their sleeves and get the life-saving vaccine.”
There are still two more steps to go before final approval of the vaccine for children. By the end of the week, the FDA is expected to make its decision on emergency use. Next week, the CDC will make the decision on whether to recommend the shots and who will get them.
RELATED: Moderna says their low-dose COVID-19 shot works on kids 6 to 11
RELATED: Pfizer says COVID-19 vaccine more than 90% effective in kids
SUGGESTED VIDEOS: Latest from 9NEWS