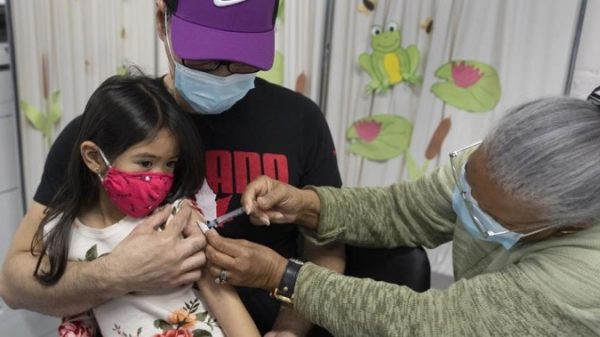
The Centers for Disease Control and Prevention (CDC) approved the use of Pfizer’s COVID-19 vaccine for children aged five to 11 Tuesday, allowing physicians across the United States to begin administering the shot to school-aged children.
The approval comes after an advisory committee to the CDC recommended on Tuesday that Pfizer’s shots be given to this age group.
The advisers said the benefits of vaccination outweigh the risks of the vaccine. Much of their discussion stemmed from rare cases of heart inflammation that have been linked to the vaccine, particularly in young men.
CDC Director Rochelle Walensky signed off on the recommendations, saying it was an “important step” in the country’s fight against COVID-19.
“We know millions of parents are eager to get their children vaccinated and with this decision, we now have recommended that about 28 million children receive a COVID-19 vaccine,” Walensky said in a statement.

U.S. President Joe Biden called it a “major step forward”.
“It will allow parents to end months of anxious worrying about their kids, and reduce the extent to which children spread the virus to others,” Biden said in a statement.
Physicians already had millions of doses of Pfizer’s pediatric vaccine — which is a dosage one-third of the strength of the version given to adults — shipped to their offices and ready to be doled out, after the Food and Drug Administration (FDA) gave emergency authorization to the vaccine last week.
The U.S. has plans to scale up the distribution to full capacity starting next week, the CDC said.
Read more:
U.S. shipping millions of COVID-19 vaccines for younger kids ahead of CDC decision
The vaccine consists of two doses to be given three weeks apart.
According to the trials, Pfizer’s pediatric shots were nearly 91 per cent effective in preventing COVID-19 among children aged 5-11 years. The side effects were mild and similar to those seen in adults and with other vaccines recommended for children, the CDC said. The most common side effect was a sore arm.
Pfizer has also applied for approval of its pediatric vaccine in Canada, though Health Canada is still reviewing the application. A decision could still be “a few weeks away,” and likely won’t come until at least mid-to-late November, Health Canada’s Dr. Supriya Sharma said at a press conference Friday.

White House coronavirus response co-ordinator Jeff Zients said Monday the United States has enough supply of the Pfizer/BioNTech vaccine for all 28 million children aged five to 11. While some children may be able to get their first shots as soon as Wednesday, Zients said the U.S. pediatric vaccine program will be running at full strength by next week.
The FDA authorized a 10-microgram dose of Pfizer’s vaccine in young children. The original shot given to those age 12 and older is 30 micrograms.
Read more:
Pfizer COVID-19 vaccine authorized for children 5-11, U.S. FDA says
Advisers to the FDA last week said the lower dose could help mitigate some of the rare side effects. At their meeting, they paid close attention to the rate of a heart inflammation called myocarditis that has been linked to the vaccines from Pfizer/BioNTech and Moderna, primarily in young men.
Children are also at lower risk of hospitalization from COVID-19 infection than older groups.
Few countries so far have authorized the Pfizer vaccine in this five to 11 age group. Bahrain also approved it on Tuesday.
— with files from Reuters
View link »
© 2021 Global News, a division of Corus Entertainment Inc.